Kengrexal is the first and only intravenous P2Y12 inhibitor providing rapid platelets inhibition during PCI1
Kengrexal is the only P2Y12 inhibitor with3:
• Onset within 2 minutes1
• Offset within 1 hour after discontinuation1
• Average elimination half-life of 3–6 minutes1
Indication:
Kengrexal, co-administered with acetylsalicylic acid (ASA), is indicated for the reduction of thrombotic cardiovascular events in adult patients with coronary artery disease undergoing percutaneous coronary intervention (PCI) who have not received an oral P2Y12 inhibitor prior to the PCI procedure and in whom oral therapy with P2Y12 inhibitors is not feasible or desirable.1
Important safety information1
Kengrexal for injection is contraindicated in patients with:
- Active bleeding or increased risk of bleeding, because of impaired haemostasis and/or irreversible coagulation disorders or due to recent major surgery/trauma or uncontrolled severe hypertension.
- History of stroke or transient ischemic attack (TIA).
- Known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.1
The most common adverse reactions with cangrelor include mild and moderate bleeding and dyspnoea.1
Treatment with Kengrexal may increase the risk of dyspnoea. The severity of most dyspnoea events was mild or moderate and transient.1,4
How Kengrexal works
Kengrexal is the first and only intravenous P2Y12 inhibitor.1,3
Kengrexal is a direct P2Y12 platelet receptor inhibitor that blocks ADP-induced platelet activation and aggregation. It binds selectively and reversibly to the P2Y12 receptor to prevent further signaling and platelet activation.
Rapid onset within 2 minutes1
Rapid onset of platelet inhibition within 2 minutes:
- Intravenous administration.
- Direct acting.
Rapid offset within 1 hour1
Reversible, with rapid offset and return of platelet function within 1 hour after discontinuation:
- Patients who need CABG or surgery after PCI can proceed soon after Kengrexal discontinuation.
- Average elimination half-life of 3–6 minutes.
- Metabolism independent of organ function.
Consistent pharmacokinetics1
Not influenced by:
- Age
- Gender
- Renal status
- Hepatic function
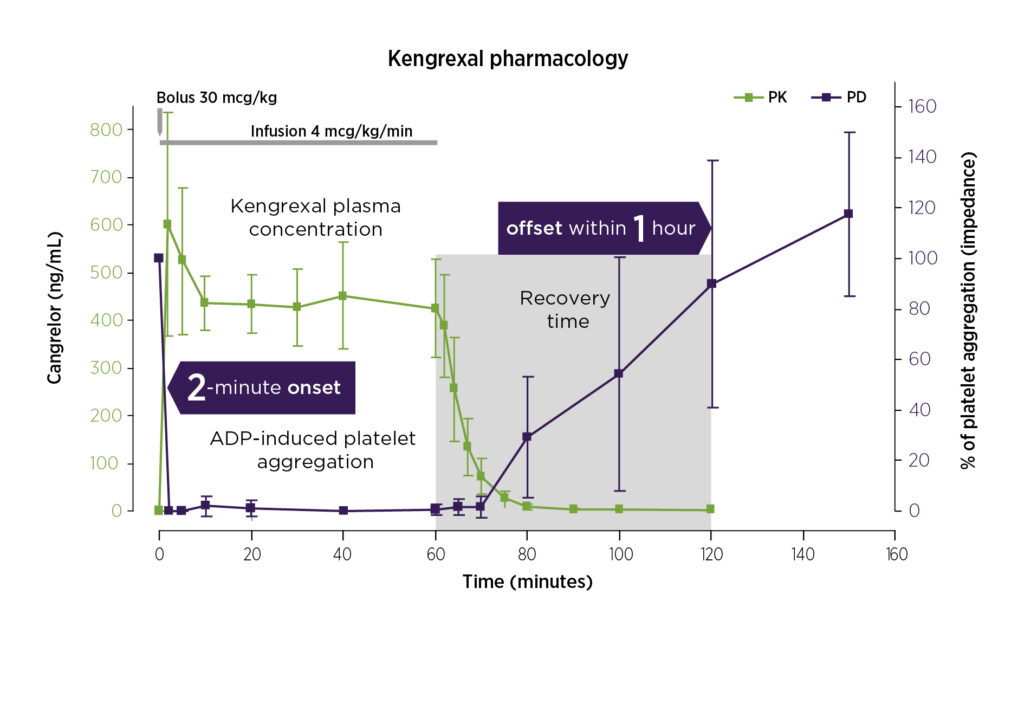
Blood levels of Kengrexal (green line/PK), and platelet activity (purple line/PD) with administration of a 30 mcg/kg IV bolus followed by a 4 mcg/kg/min IV infusion.2.
(Graph shows a combination of fig. 1 and fig. 2 in ref 2, and is modified by Chiesi)
Administration and dosing1

Each pack of Kengrexal includes 10 glass vials for single-use.
Kengrexal is supplied as a sterile, lyophilized powder in 10 mL single-use vials, each containing 50 mg. It must be administered as a bolus dose followed by intravenous infusion.
Reconstitution and dilution should be prepared with an antiseptic technique. Bolus and infusion—always taken from the diluted bag.
Reconstitute
Reconstitute the contents of the vial immediately before dilution.
- Add 5 mL of sterile water for injection to one 50 mg vial.
- Swirl gently until all material is dissolved; avoid vigorous mixing.
- Allow any foam to settle.
- Reconstituted Kengrexal will be a clear, colorless to pale yellow solution. Product should not contain particulate matter.
Dilute
Prior to administration, each reconstituted vial must be further diluted. Dilution can be in either 0,9 % sodium chloride solution for injection or 5 % glucose solution for injection.
- Withdraw the contents of a reconstituted vial and add 250 mL of sodium chloride 9 mg/mL (0,9 %) solution for injection or glucose solution (5 %) for injection.
- Mix carefully. Ensure that the contents of the vial are fully dissolved and the reconstituted material is a clear, colorless to pale yellow solution. Parenteral drug products should be inspected visually for particulate matter after reconstitution
- After dilution, 1 mL of solution contains 200 micrograms of Kengrexal.
- Patients weighing 100 kg or more need at least two vials in two bags.
Administration of bolus dose and intravenous infusion for PCI-procedure
Kengrexal should be administered as an intravenous bolus dose immediately followed by an intravenous infusion. The bolus and infusion should be administered from the infusion solution. The recommended dosage of Kengrexal is a 30 mcg/kg IV bolus followed immediately by a 4 mcg/kg/min IV infusion. Initiate the bolus infusion prior to PCI. The maintenance infusion should ordinarily be continued for at least 2 hours or for the duration of PCI, whichever is longer.
The dosage of Kengrexal, bolus and infusion, is based on the patient’s body weight. The dose of Kengrexal is not affected by gender, age, kidney, or liver status. See the dosing table for PCI.
Kengrexal should not be mixed with other medicines. Following administration of Kengrexal chronic treatment with P2Y12 inhibitors should be initiated.
See further in Kengrexal product summary for more information.
Clinical documentation and safety
CHAMPION PHOENIX study design4
CHAMPION PHOENIX was a randomized, double-blind, placebo-controlled, phase III trial in 11,145 patients who were undergoing either urgent or elective PCI and were receiving guideline-recommended therapy. Patients received a bolus and infusion of Kengrexal or a loading dose of 600 mg or 300 mg of clopidogrel.4
CHAMPION PHOENIX phase III trial efficacy results1,4
As an adjunct to PCI, Kengrexal significantly reduced the primary composite endpoint events of death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis at 48 hours vs clopidogrel in an all-comer PCI patient population. The relative risk reduction for the Kengrexal arm was 22% (absolute risk reduction 1,2% (5,9% vs 4,7%, OR 0,78, CI 0,66-0,93, p=0,005)1
Kengrexal showed significant reductions in periprocedural thrombotic complications compared to clopidogrel1,4
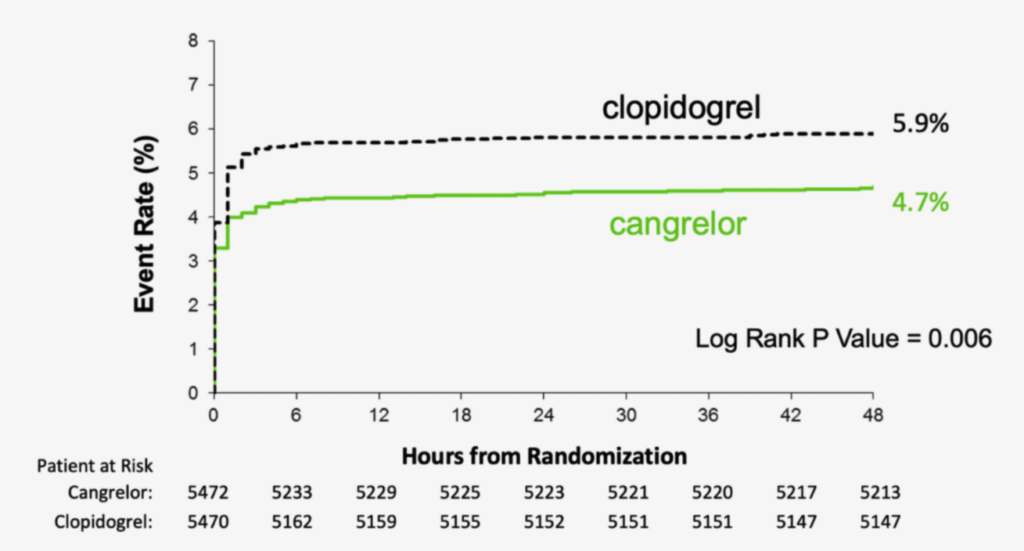
CHAMPION PHOENIX primary composite endpoint—MACE (death, MI, IDR, stent thrombosis at 48 hours): Kengrexal vs clopidogrel in an all-comer PCI cohort.1,4 Graph is an excerpt of full scale, as indicated in figure 1 Bhatt et al 2013.
Patients receiving Kengrexal had no statistically significant increase in either GUSTO-defined severe bleeding or need for transfusion.1,4
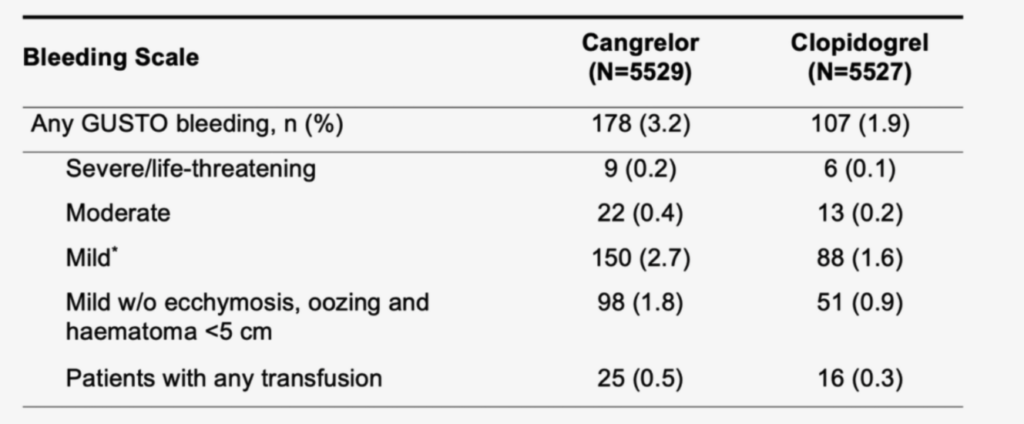
Major Bleeding Events (Non-CABG-related) at 48 Hours.
GUSTO = Global Use of Strategies to Open occluded Arteries; CABG = Coronary Artery Bypass Grafting.
* In CHAMPION PHOENIX, GUSTO Mild was defined as other bleeding requiring intervention but not requiring blood transfusion or causing haemodynamic compromise.
PCI patient cases
Learn more from PCI patient cases. Dr Per Grimfjärd, MD, PhD., takes you through three patient cases considering different treatments, including cangrelor.
References:
1. Kengrexal SPC, 02/2026.
2. Akers WS, Oh JJ, Oestreich JH, Ferraris S, et al. Pharmacokinetics and Pharmacodynamics of a Bolus and Infusion of Cangrelor. J Clin Pharmacol 2010;50:27–35.
3. www.legemiddelsok.no
4. Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events – Champion Phoenix. N Engl J Med 2013;368:1303–13.
Kengrexal (kangrelor)
50 mg pulver til konsentrat til infusjons-/injeksjonsvæske, oppløsning
Indikasjon: Reduksjon av trombotiske kardiovaskulære hendelser hos voksne med koronararteriesykdom, som gjennomgår perkutan koronar intervensjon (PCI), som ikke har fått en oral P2Y12-hemmer før PCI-prosedyren, og som oral P2Y12-behandling ikke er ønskelig eller gjennomførbart for. Administreres sammen med ASA.
Dosering: Anbefalt dose: 30 µg/kg som i.v. bolus, umiddelbart etterfulgt av 4 µg/kg/minutt som i.v. infusjon. Bolusen og infusjonen skal innledes før prosedyren, og fortsettes i minst 2 timer eller så lenge prosedyren varer, det som måtte vare lengst. Etter legevurdering kan infusjonen fortsettes i totalt 4 timer. Skal administreres av lege med erfaring i enten akutt koronarpleie eller i koronar-intervensjonsprosedyrer, og er ment for spesiell bruk i akutt- eller sykehusmiljø.
Pakninger og pris (AUP): 10 stk. hetteglass: kr 37 623,50. Reseptgruppe: C.
Utvalgt sikkerhetsinformasjon
- Kontraindisert ved aktiv blødning eller økt risiko for blødning pga. forringet hemostase og/eller irreversible koagulasjonssykdommer, eller pga. nylig, omfattende kirurgi/traume eller ukontrollert alvorlig hypertensjon, samt ved enhver historikk med slag eller transitorisk iskemisk anfall.
- Kan øke blødningsrisikoen, inkl. intrakraniell blødning.
- Brukes med forsiktighet ved sykdomstilstander forbundet med økt blødningsrisiko, og ved bruk av legemidler som kan øke blødningsrisikoen.
- Kan øke risikoen for hjertetamponade og dyspné.
- Skal brukes med forsiktighet ved alvorlig nedsatt nyrefunksjon.
- Graviditet og amming: Anbefales ikke under graviditet. En risiko for nyfødte/spedbarn som ammes kan ikke utelukkes.
- Bivirkninger: Vanligste er lett og moderat blødning og dyspné. Alvorlige inkluderer alvorlig/livstruende blødning og overfølsomhet.
For utfyllende informasjon om dosering, kontraindikasjoner, advarsler og forsiktighetsregler, interaksjoner og bivirkninger se Kengrexal SPC, godkjent 02/2026.
